Revolutionary HIV Treatment: Lenacapavir Achieves 100% Clinical Efficacy
In 2024, the journal Science recognized Lenacapavir as the “Breakthrough of the Year”, marking a transformative milestone in the fight against HIV. This innovative injectable drug has demonstrated unprecedented effectiveness in preventing HIV infection, providing six months of protection with a single injection. Its potential to significantly reduce global infection rates offers new hope for individuals and communities affected by the virus.
How Does Lenacapavir Work?
Unlike other HIV treatments, Lenacapavir targets the HIV capsid, a conical structure that protects the virus’s genetic material. The drug reinforces the capsid, disrupting crucial processes such as viral replication and nuclear integration in infected cells. This unique mechanism effectively blocks the virus from multiplying, marking a groundbreaking shift in HIV prevention strategies.
Lenacapavir Dosage Frequency
Currently, Lenacapavir requires just two injections per year to maintain effective pre-exposure prophylaxis (PrEP). This biannual dosing schedule represents a major improvement in convenience compared to daily oral medications or bimonthly injections.
The manufacturer, Gilead Sciences, is actively researching ways to extend the drug’s duration to a full year per dose, though this remains in the experimental phase.
Clinical Trial Success
Two landmark clinical trials in 2024 highlighted Lenacapavir’s effectiveness:
-
Study on Women and Adolescents in Africa:
- Conducted in South Africa and Uganda, involving over 5,000 transgender participants.
- Zero cases of HIV infection were reported among those receiving Lenacapavir, achieving a 100% success rate.
-
Multinational Study:
- Over 2,000 participants of various genders from South America, Asia, Africa, and the U.S.
- Achieved a 99.9% success rate, with only two cases of infection recorded.
In the PURPOSE-2 trial (2024), Lenacapavir reduced HIV risk by 96% when administered every six months. Among 2,180 participants, only two infections occurred, compared to nine infections in the 1,087 participants taking daily oral PrEP (Tenofovir/Emtricitabine).
Health experts describe these results as "unprecedented", solidifying Lenacapavir as one of the most promising HIV prevention tools available.
How Lenacapavir Compares to Other Treatments
- Fewer Doses Required: Traditional PrEP requires daily pills, while injectable PrEP is taken every two months—Lenacapavir reduces this to just two injections per year.
- Higher Efficacy: Clinical trials indicate Lenacapavir is significantly more effective, particularly among high-risk populations.
- Innovative Approach: This breakthrough may pave the way for similar treatments targeting other viral diseases.
Challenges to Overcome
Despite its groundbreaking success, Lenacapavir faces several hurdles before it can become a global solution:
-
Cost and Accessibility:
- Agreements have been made to manufacture generic versions in 120 developing nations.
- However, pricing and distribution remain unclear in middle-income countries like Brazil.
-
Healthcare Infrastructure:
- In regions with limited healthcare access, distributing injectable treatments may pose logistical challenges.
-
Social Acceptance:
- The stigma surrounding HIV and its treatment may discourage individuals from adopting this preventive solution.
Lenacapavir and the Future of HIV Prevention
While Lenacapavir marks a revolutionary step forward, it does not replace the need for an HIV vaccine. A vaccine would provide long-term protection for everyone, regardless of risk factors. However, until that becomes a reality, Lenacapavir has the potential to save millions of lives by dramatically reducing HIV transmission rates worldwide.
WHO's Perspective
The World Health Organization (WHO) has enthusiastically welcomed the clinical trial results, emphasizing Lenacapavir’s high effectiveness in preventing HIV, particularly among women and adolescents in Africa. WHO is actively developing guidelines to facilitate the global rollout of this treatment in public health programs.
Conclusion
Lenacapavir is not just a medical breakthrough—it represents new hope for those at high risk of HIV infection. Its exceptional efficacy, combined with the convenience of two injections per year, makes it a game-changer in the fight against the HIV epidemic. However, to fully realize its potential, global efforts must focus on ensuring accessibility, overcoming distribution challenges, and breaking down social stigmas.
This remarkable innovation brings us one step closer to a future without HIV.
News in the same category


42-Day Juice Therapy – Natural Cancer Support Discovered by Rudolf Breuss

5-Year-Old Girl Dies from Late-Stage Cancer — A Wake-Up Call for All Parents

Parsley Leaf Tea

Why Non-Smokers Can Still Get Lung Cancer

Salmonella Outbreak Linked to Recalled Eggs Sickens 79 People Nationwide, Including 2 in New Jersey

Top 10 Foods That Reduce Your Uric Acid Levels

Just Three Nights of Poor Sleep May Harm Your Heart, New Study

PINEAPPLE – A NATURAL REMEDY FOR STROKE PREVENTION & LIVER-KIDNEY DETOX

This 2025 Herbal Elixir Is Emptying Hospitals—What’s Behind the Craze?
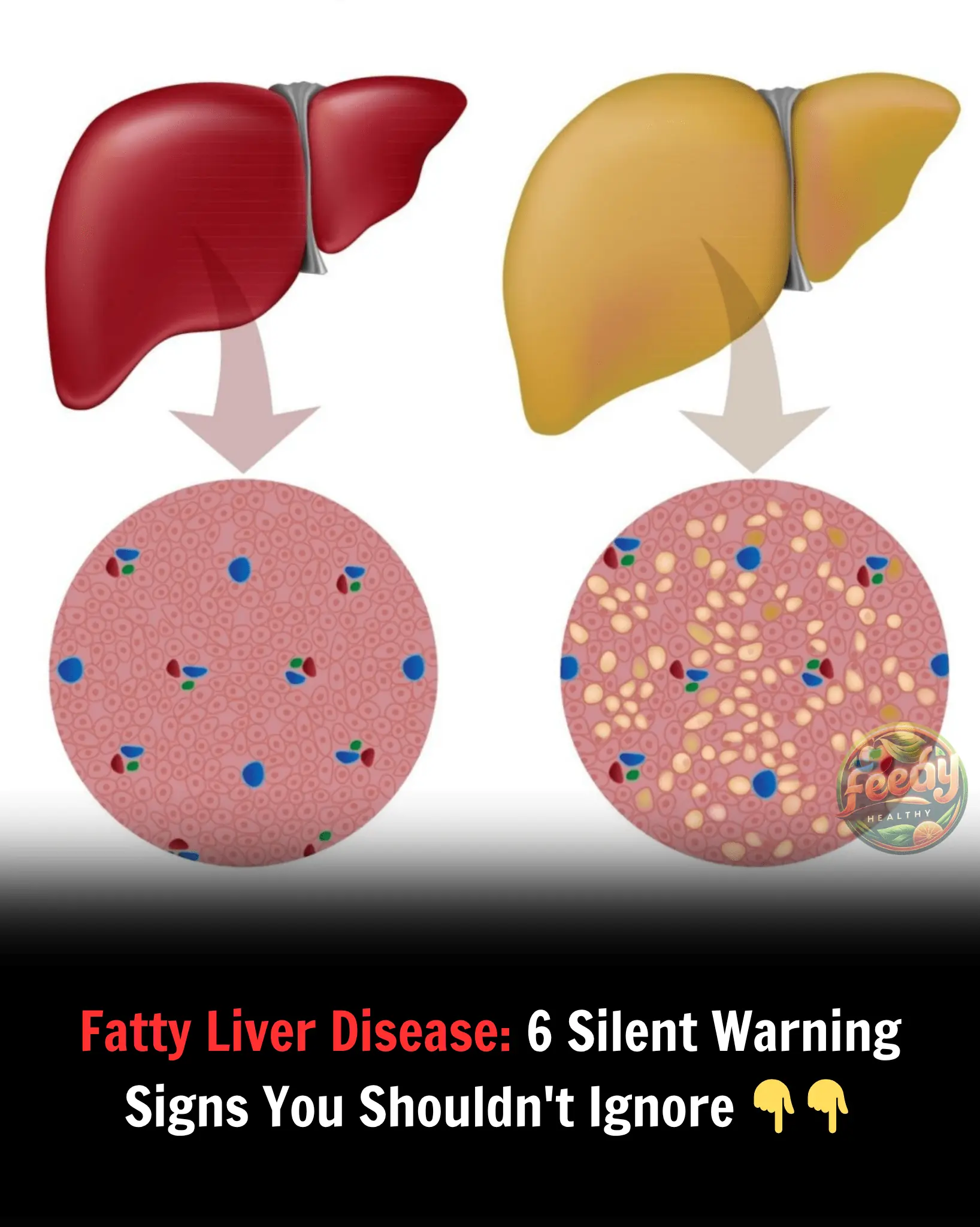
Fatty Liver Disease: 6 Silent Warning Signs You Shouldn't Ignore 👇👇

11 AM – THE GOLDEN HOUR WHEN YOUR BLOOD FAT AND BLOOD PRESSURE WILL “BE GRATEFUL” IF YOU REMEMBER TO DO THIS ONE SIMPLE THING…

Just One Spoonful of This Mixture Can Help with Blindness, Myopia, Blurred Vision, and Eye Fatigue

Mimosa Pudica: The Sensitive Plant That May Boost Your Health Naturally

Say Goodbye to Joint Pain, Arthritis, and Rheumatism in Just 3 Days – Try This Potent Clove and Garlic Remedy!

What Should People with Weak Kidneys Avoid in Their Daily Meals?

6 Powerful Juice Recipes to Naturally Help Anemia, Inflammation, Fatigue, Low Immunity, High Cholesterol, and Insomnia

The Natural Remedy Called “The Cancer Eliminator”: A Simple Blend for Better Health and Vision
Molecular Jackhammers: A Breakthrough in Non-Toxic Cancer Treatment Using Near-Infrared Light
6-Year-Old Boy Dies from Late-Stage Cancer: A Wake-Up Call About Sugary Juice Consumption
News Post

If you drink cucumber water every morning, this is what happens to your body

I Used to Suffer from Diabetes, Poor Circulation, and High Blood Pressure — Until a Natural Healer Shared This Recipe, and It Changed My Life Completely

Mixing Papaya with Flaxseed Can Help Flatten Your Belly, Relieve Constipation, and Soothe an Irritated Colon

The Drink That Eliminates Kidney Stones in the Blink of an Eye

THE NATURAL DRINK THAT LOWERS TRIGLYCERIDES, BALANCES BLOOD SUGAR, AND REDUCES BELLY BLOAT

Garlic, Lemon Juice, Grated Ginger, and Honey: A Powerful Natural Remedy 🍋✨

Healthy Flourless & Sugar-Free Energy Cookies!

THE NATURAL DRINK SHARED BY DR. FRANK SUÁREZ
Garlic Can Kill 14 Different Infections – I Bet You Didn’t Know This!

A Powerful Natural Blend for Everyday Wellness

What Happens When You Put Vinegar on Your Feet?

GINGER – THE MOST POWERFUL ROOT FOR ENERGY, IMMUNITY, AND HEALING

Unleash the Power of Banana Peels: Surprising Uses You Need to Know

Discover the Magical Benefits of Mint and Honey!

Natural Remedy to Lower Blood Sugar, Cleanse Kidneys, and Reduce Cholesterol

REMEDY FOR BLURRY VISION, CATARACTS, MYOPIA, AND MACULAR DEGENERATION 👁️
THE PLANT THAT ELIMINATES COUGH, PHARYNGITIS, LARYNGITIS, BRONCHITIS, AND TUBERCULOSIS

Discover the Magic of Baking Soda for Your Feet 🦶✨

THE MONEY PLANT
