
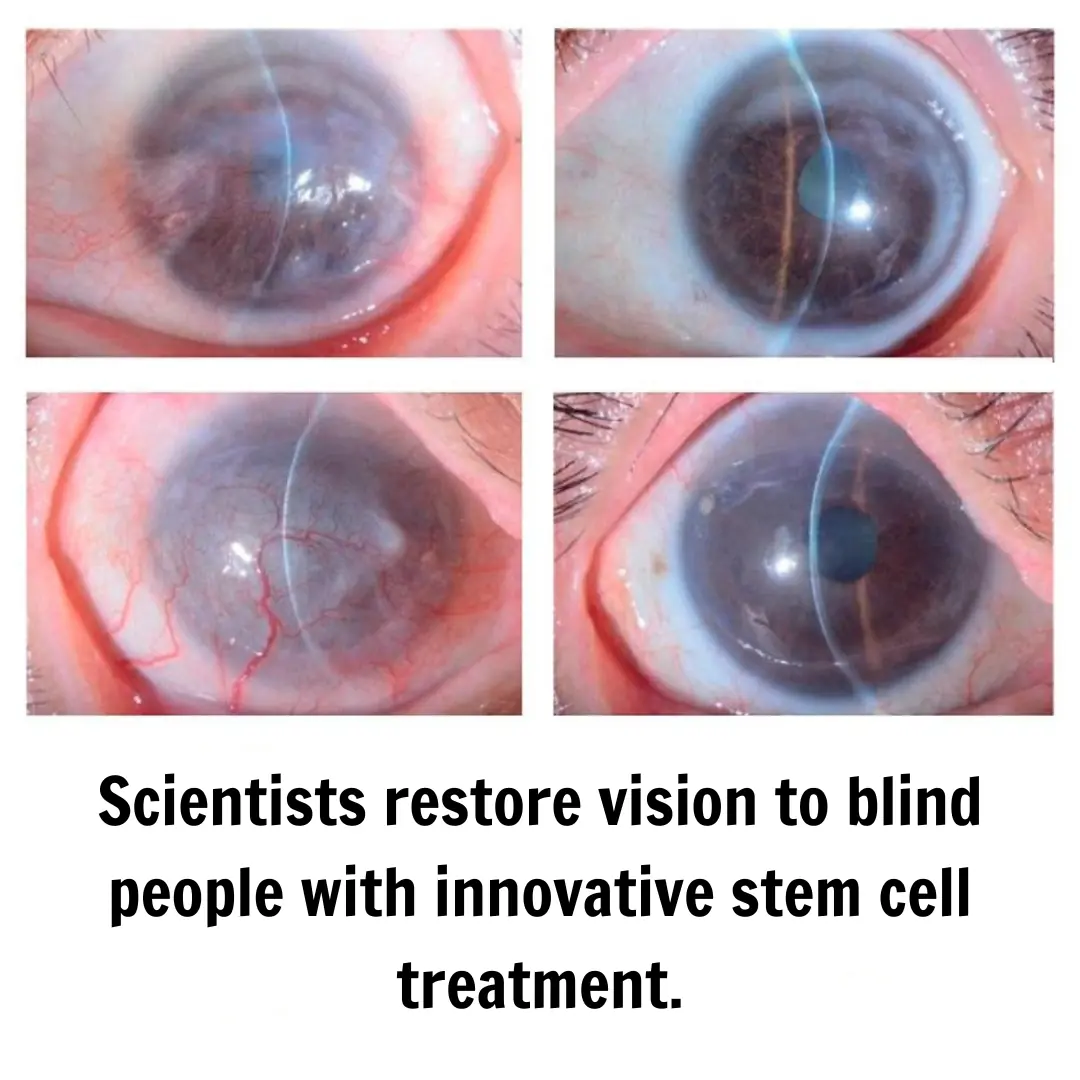
Japanese Scientists Restore Vision in Blind Individuals
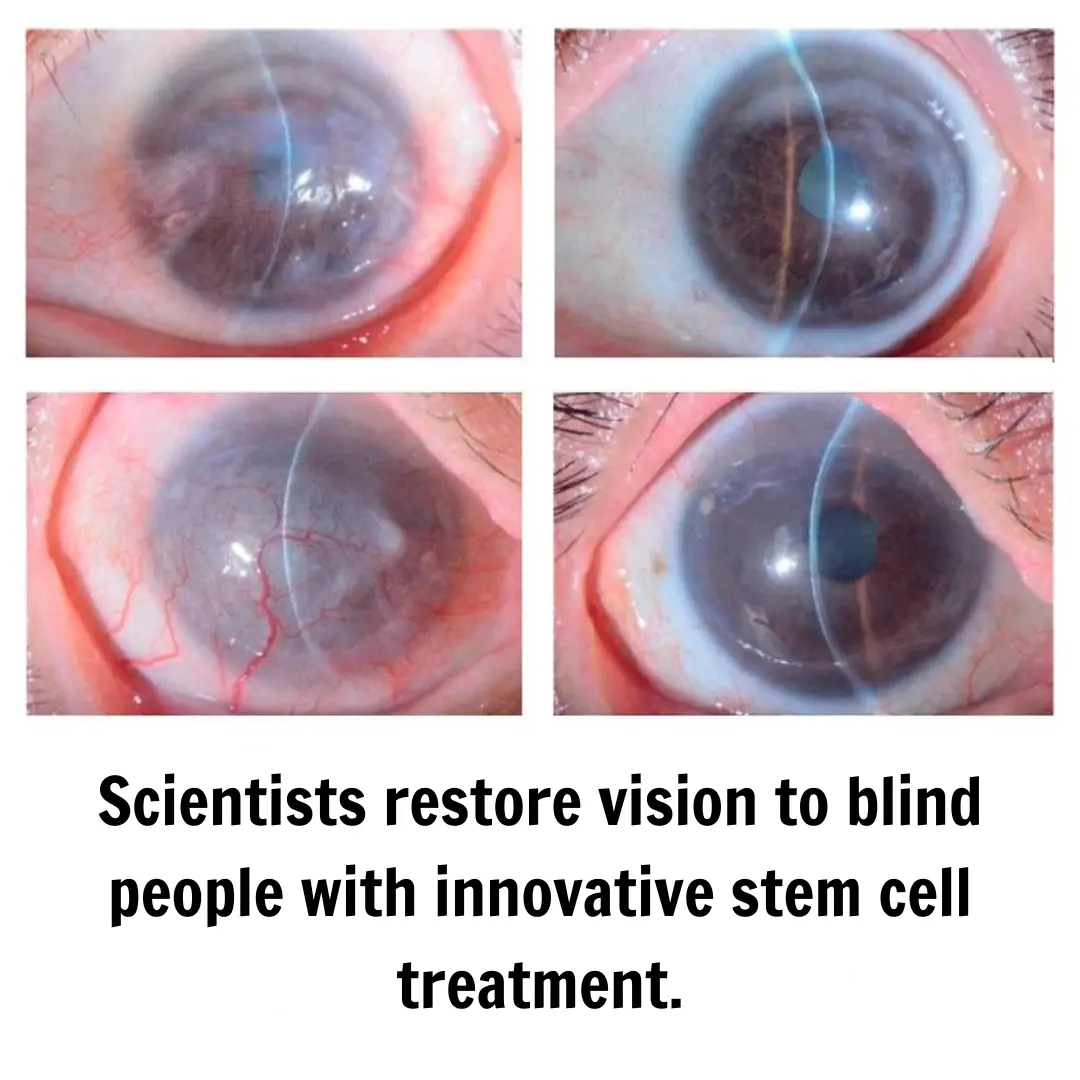
Medical science continues to advance with discoveries that once seemed like science fiction. This time, researchers in Japan have successfully restored partial vision in individuals with blindness caused by limbal stem cell deficiency (LSCD).
The cornea, the transparent layer covering the front of the eye, is crucial for clear vision. Its maintenance depends on stem cells located in the limbal region, a transition zone between the cornea and the sclera. When these cells are damaged or lost—a condition known as limbal stem cell deficiency (LSCD)—the cornea loses transparency due to the growth of fibrous tissue and blood vessels, which can lead to blindness.
This disorder can result from chemical burns, autoimmune diseases, or chronic infections. Until now, treatments included corneal transplants or grafts of cultured tissue from the patient’s own body, but these methods have limitations: donor shortages, the risk of immune rejection, and challenges in treating bilateral cases.
Treating Blindness with iPSC Stem Cells
Induced pluripotent stem cells (iPSCs) have emerged as a promising alternative for addressing diseases that previously had no definitive solution. Unlike embryonic stem cells, iPSCs are derived from adult cells (such as skin or blood cells) and genetically reprogrammed to acquire a pluripotent state. In other words, they regain the ability to differentiate into nearly any cell type in the body.
Read More: First Successful Human Eye Transplant in History
This method significantly reduces the ethical concerns associated with embryo use and, most importantly, minimizes the risk of immune rejection, as the material is potentially derived from the patient’s own body. Specifically for the cornea, these induced pluripotent stem cells have enabled the creation of functional corneal epithelia, opening the door to new ocular regeneration therapies.
Corneal Epithelium Transplant Derived from iPSCs
A recent study published in the prestigious medical journal The Lancet describes the first clinical trial conducted in Japan using induced pluripotent stem cells to reconstruct the corneal epithelium in patients with blindness caused by LSCD. This research represents not only a groundbreaking biomedical achievement but also demonstrates the feasibility and safety of the procedure in humans.
Methodology
🔹 Cellular Reprogramming:
A line of iPSCs was obtained from umbilical cord blood donors. These cells were then cultured under special conditions that promoted their differentiation into corneal epithelial cells.
🔹 Epithelium Fabrication:
Once differentiated, the corneal epithelial cells were organized into sheets known as iPSC-derived corneal epithelial cell sheets (iCEPS). These sheets exhibited characteristics of natural corneal epithelium, such as the expression of specialized proteins.
🔹 Transplantation:
Four patients with different causes of limbal stem cell deficiency underwent surgery to remove the fibrous tissue covering the cornea. The iCEPS were then carefully sutured onto the ocular surface and protected with a therapeutic lens.
🔹 Follow-up:
Over the 52 weeks following surgery, researchers monitored the condition of the cornea, assessing transparency, visual acuity, and the absence of rejection or severe complications. The follow-up period extended up to two years to rule out long-term adverse events.
Results: Restoring Vision
Findings revealed that after surgery, patients experienced a significant improvement in corneal clarity and visual acuity. In three out of four cases, the regenerated corneal epithelium remained stable throughout the follow-up period, with no signs of rejection or severe inflammation. One patient showed initial progress, but part of the tissue deteriorated slightly over time, highlighting the need for further studies to optimize the therapy.
A particularly notable outcome was the absence of serious adverse events, such as tumor formation or intense immune reactions. The use of iPSCs, which tend to express lower levels of HLA molecules than conventional cells, may have contributed to a reduced risk of rejection. Additionally, researchers noted that the graft did not contain immunocompetent cells, decreasing the likelihood of uncontrolled inflammatory responses.
Another key aspect was the improvement in patients' quality of life and subjective perception. Self-assessment surveys indicated greater independence in daily tasks and reduced eye irritation. This demonstrates the potential effectiveness of this approach not only in anatomical restoration but also in enhancing patients' everyday experiences.
The Future of iPSC Therapy in Ophthalmology
The implementation of iPSC-based therapies to regenerate the corneal epithelium marks a turning point in ophthalmology. Here are some future projections:
🔹 Expanded Clinical Trials: The next logical step is to conduct studies with a larger number of participants and a broader range of cases. This will help confirm large-scale effectiveness, refine production protocols, and lower costs.
🔹 Applications Beyond the Cornea: While the current focus is on limbal stem cell deficiency, iPSC research could extend to treating other conditions, such as retinal degeneration or glaucoma.
🔹 Optimization of Manufacturing: Mass production of iCEPS with high quality and safety standards is crucial. Automated culture technologies and 3D bioprinting are expected to accelerate the availability of these grafts for a larger patient population.
🔹 Reduced Dependence on Immunosuppressants: This trial suggests the possibility of limiting the use of immunosuppressive drugs, especially in iCEPS that do not trigger severe immune responses. However, more research is needed to determine the minimum effective dose and long-term safety profile.
Conclusions
The pioneering work of Japanese scientists demonstrates that restoring vision in individuals with limbal stem cell deficiency through iPSC-derived corneal epithelial transplants is both feasible and safe over a follow-up period of up to two years. This research paves the way for new treatment possibilities for patients with corneal blindness, addressing some of the major obstacles of traditional methods, such as donor shortages and the risk of immune rejection.
News in the same category


Scandalous discovery of why intimate parts smell like fish

First childhood death from measles reported in the US amid active outbreak.
10 Foods That You Should Eat Daily For Clean Arteries

10+ Foods to Help Lower Your Blood Sugar

5 Types of Foods That Naturally Contain Progesterone – Doctor’s Advice: Women Over 45 Should Eat More
What Happens When Pancreatic Cancer Is Caused by Diet? Doctors Warn: People with a Weak Pancreas Should Avoid These Foods

Meet Helicobacter pylori: A Dangerous Bacteria That Can Cause Deadly Cancer
Scientists Successfully Rejuvenate a 53-Year-Old Woman’s Skin Cells by 30 Years

New Highly Contagious and Virulent HIV Variant Discovered
Japanese Scientists Restore Vision in Blind Individuals
The First Lung Cancer Vaccine Enters Clinical Trial Phase in Seven Countries

Is Eating Sweet Potatoes “Anti-Cancer” or “Cancer-Promoting”? Research Findings Have Been Released
Can You Eat Onions If You Have High Blood Pressure? Doctor’s Advice: Avoid These 3 Foods for Better Blood Pressure Control
Are There Warning Signs in the Body Before Cancer Appears? Check Yourself and Get Screened as Soon as Possible
Kidney Cancer: Warning Signs You Should Not Ignore

🌿 Mimosa Pudica: The Mysterious Healing Plant of South America

Doctor's Warning: People Prone to Pancreatic Cancer Share These Common Traits – Be Careful!

Rain Water Solar Term: Remember to Eat These 2 Foods to Expel Dampness from Your Body
News Post

Excessive consumption of green juices can cause oxalate kidney injury.
10 Ways To Support Your Lymphatic System

Pure Magic: Burning a Clove of Garlic, What Happens After 15 Minutes at Home?
The Juice That Strengthens Bones and Soothes Knee Pain Naturally
Clean Your Kidneys, Liver & Lungs – Powerful Natural Detox! 🍊🌿

Euphorbia Hirta: Nature's Remedy for Respiratory and Immune Health

Why is it said that warm lemon juice can save a life: Drinking it at the right time will prevent cancer, filter blood, and remove belly fat

Scandalous discovery of why intimate parts smell like fish

First childhood death from measles reported in the US amid active outbreak.
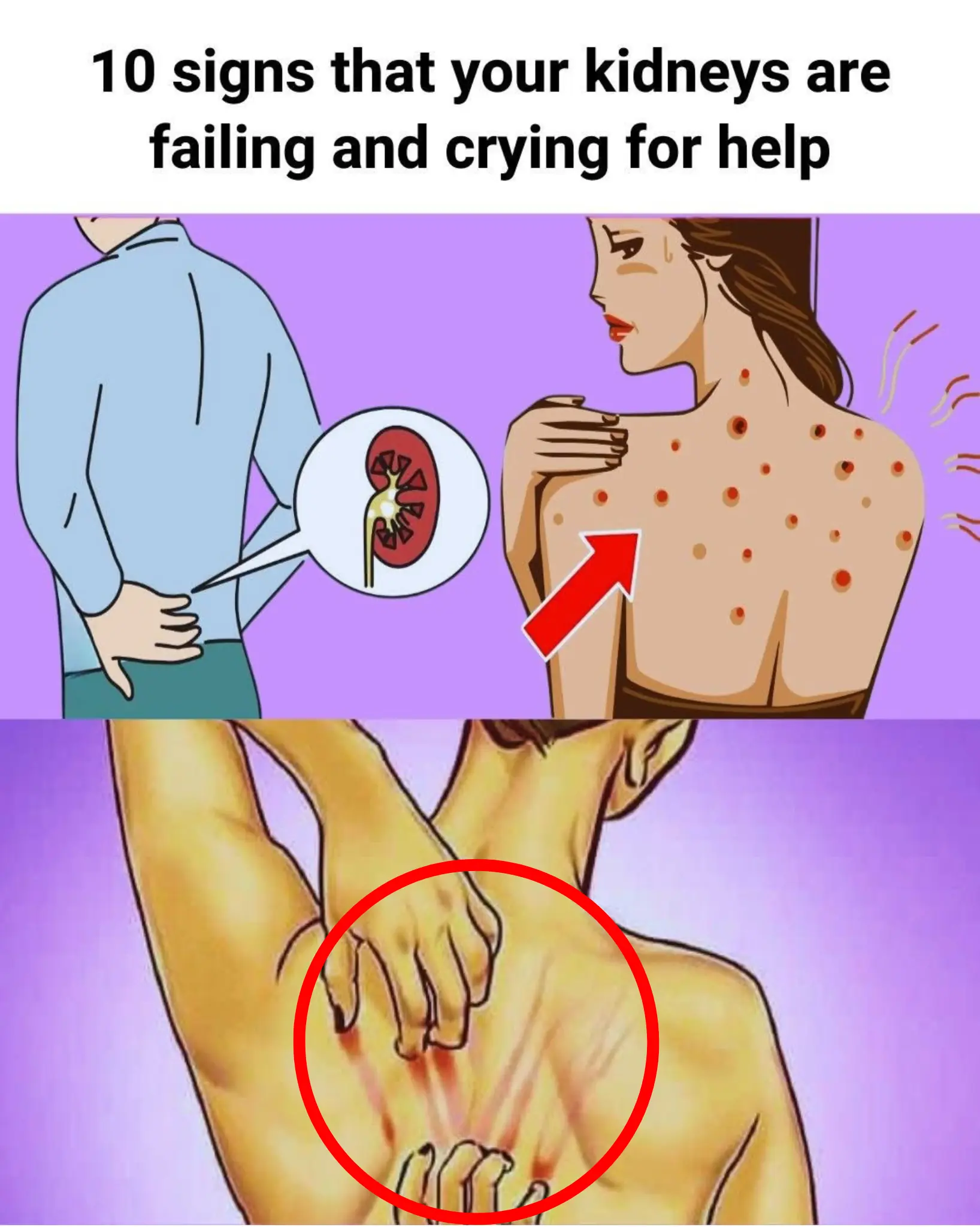
If Your Kidneys Are in Danger, the Body Will Show these 10 Signs

10 Foods That You Should Eat Daily For Clean Arteries

Natural Recipe with Watermelon, Carrot, Beetroot, and Ginger

10+ Foods to Help Lower Your Blood Sugar

5 Types of Foods That Naturally Contain Progesterone – Doctor’s Advice: Women Over 45 Should Eat More
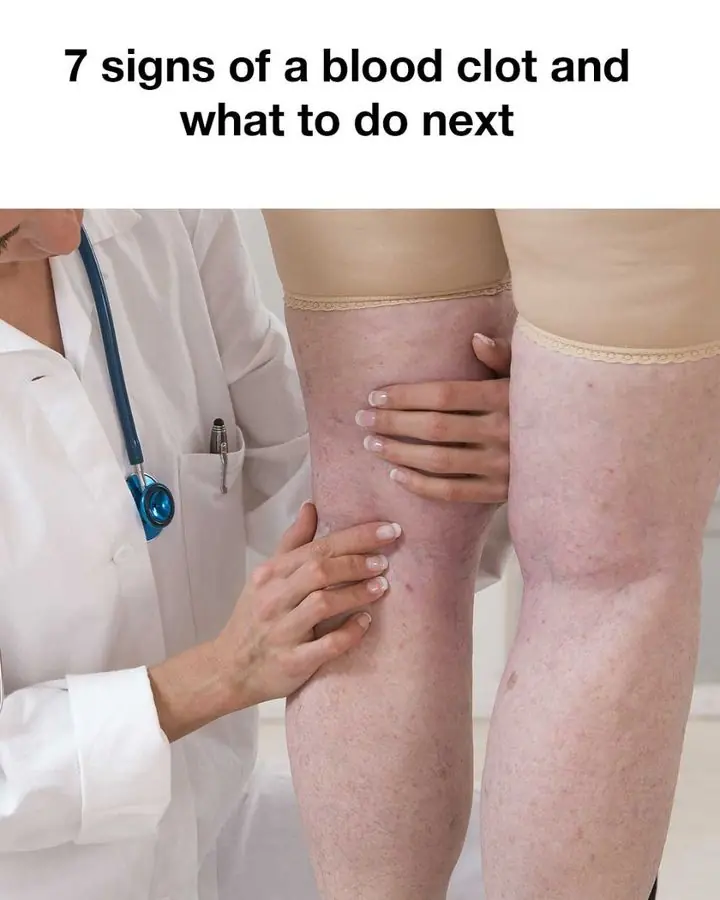
So useful! Gonna watch out for these Holly Owens Contributing Writer
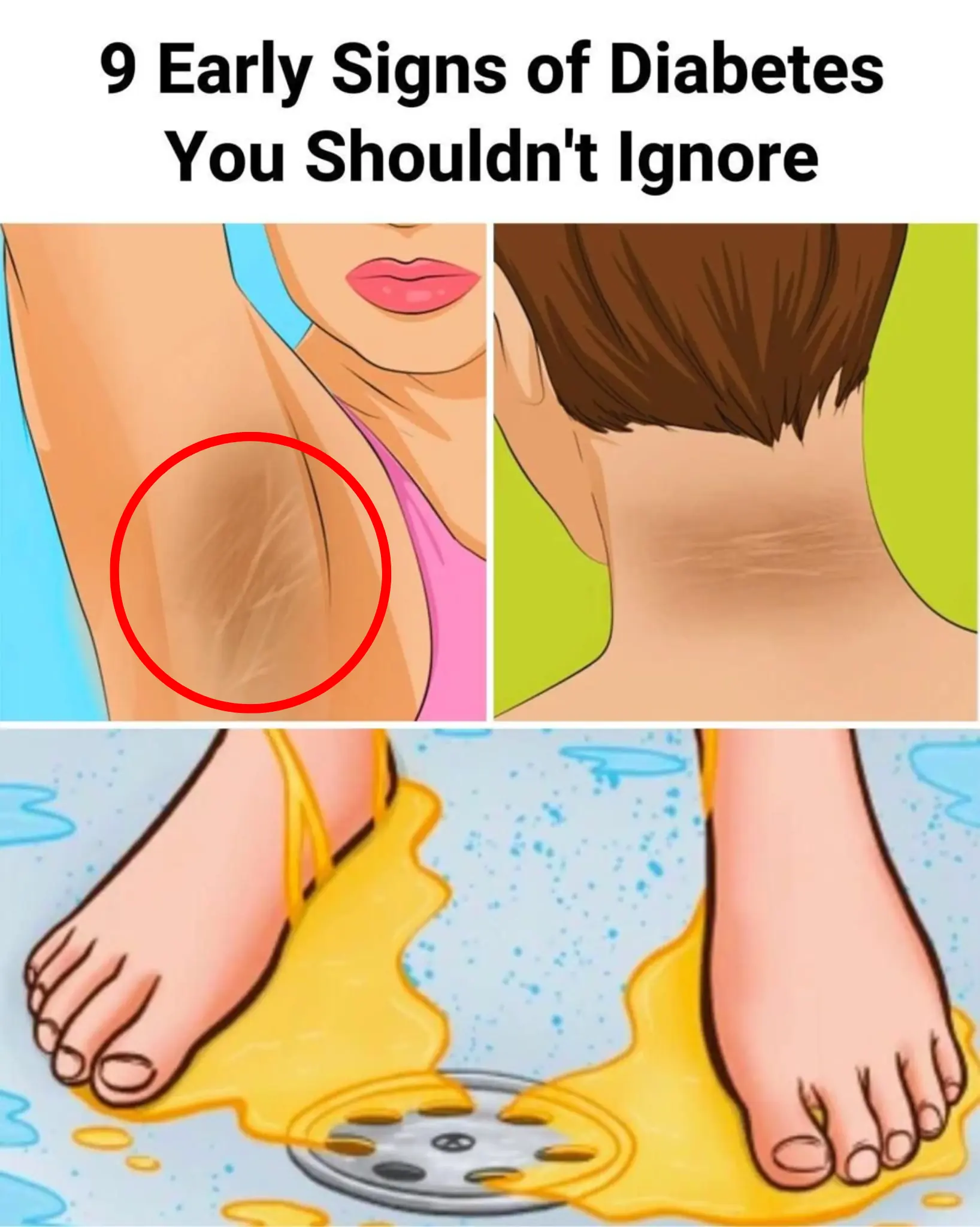
9 Early Signs of Diabetes You May Not Be Noticing
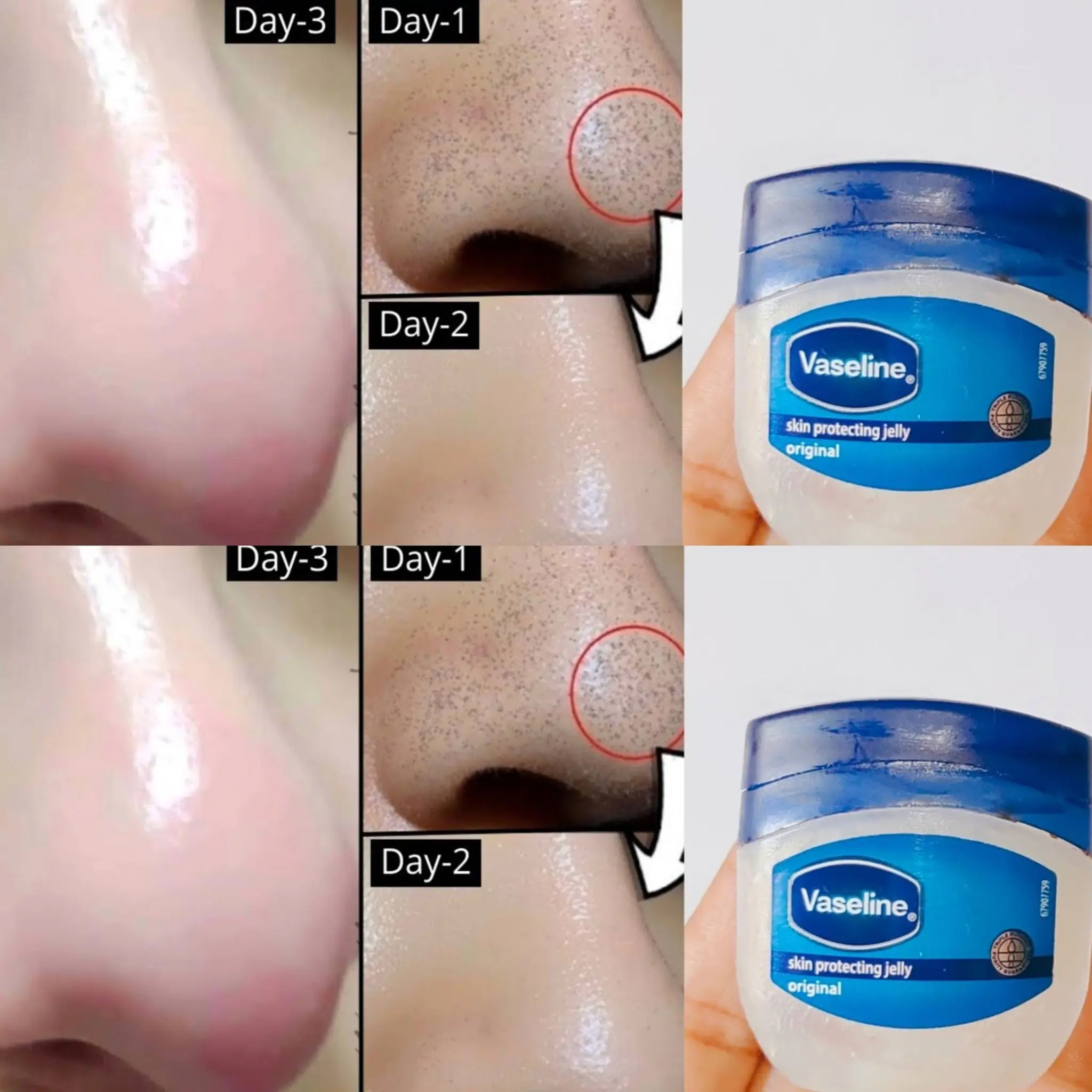
How to Remove Blackheads and Whiteheads Naturally with Vaseline

What Happens When Pancreatic Cancer Is Caused by Diet? Doctors Warn: People with a Weak Pancreas Should Avoid These Foods

Mix Avocado Seed with Turmeric and Cinnamon
