
The Mpemba Effect: Why Hot Water Can Freeze Faster Than Col

At first glance, the idea that hot water can freeze faster than cold water seems to defy logic and everything we learned in basic science. Yet, this counterintuitive phenomenon, known as the Mpemba Effect, has puzzled scientists and intrigued curious minds for decades. But what exactly is the Mpemba Effect, and why does it happen?
🔬 What Is the Mpemba Effect?
The Mpemba Effect refers to the observation that, under certain conditions, hot water freezes faster than cold water. It is named after Erasto Mpemba, a Tanzanian student who first noticed and documented the effect in the 1960s while trying to freeze ice cream mixtures. Despite being initially dismissed, his observations were later supported by physicist Denis Osborne, and the phenomenon began attracting scientific attention.
🧊 Is It Real?
Yes—but with a caveat. The Mpemba Effect doesn’t occur under all circumstances, and it is highly sensitive to environmental factors such as:
-
Initial water temperature
-
Ambient temperature
-
Container shape and material
-
Volume of water
-
Air circulation inside the freezer
In other words, hot water can freeze faster than cold, but only in specific setups where a unique combination of variables comes into play.
🧪 Possible Explanations
Despite years of experimentation, scientists still don’t agree on a single cause. Instead, several hypotheses have been proposed:
1. Evaporation
Hot water evaporates more quickly, which reduces the volume of water that needs to freeze. Less water = faster freezing.
2. Convection Currents
Warmer water sets up stronger convection currents. These help cool the water more evenly and may assist in reaching freezing temperature more quickly.
3. Supercooling Behavior
Cold water may supercool — cool below 0°C without forming ice — which delays freezing. Hot water, paradoxically, might skip this phase.
4. Dissolved Gases
Hot water contains fewer dissolved gases, and this altered composition could change the way ice forms and propagates through the liquid.
5. Frost Layer Interaction
The container holding cold water may have a frost layer that insulates it, whereas the heat from hot water may melt that layer, improving heat transfer and speeding up freezing.
🧠 Why It Still Matters
The Mpemba Effect is not just a scientific curiosity. It serves as a powerful example of how nature can surprise us, challenging assumptions and reminding us to question what we believe to be obvious. It also emphasizes the importance of observation and experimentation, even by non-experts—Erasto Mpemba being a student when he made his groundbreaking observation.
❓ Still a Mystery?
Yes. While the effect has been reproduced in laboratories, results are inconsistent. Some researchers argue that the effect may be a combination of several factors rather than a single principle. In 2016, the Royal Society of Chemistry launched a competition asking for the best theoretical explanation—illustrating that the Mpemba Effect still invites debate, even in modern scientific circles.
📌 Conclusion
The Mpemba Effect remains one of the most fascinating and debated phenomena in physics. Though we still don’t fully understand why it happens, its very existence teaches us an important lesson: science is not just about answers—it’s about asking the right questions.
So the next time you boil water, remember—you might just be watching a mystery unfold.
News in the same category


Can You Spot the 6 Hidden Words in This Living Room Image?

What’s the purpose of the fabric strip across hotel beds?

The Astonishing Life Cycle of Bamboo: A Once-in-a-Lifetime Botanical Spectacle
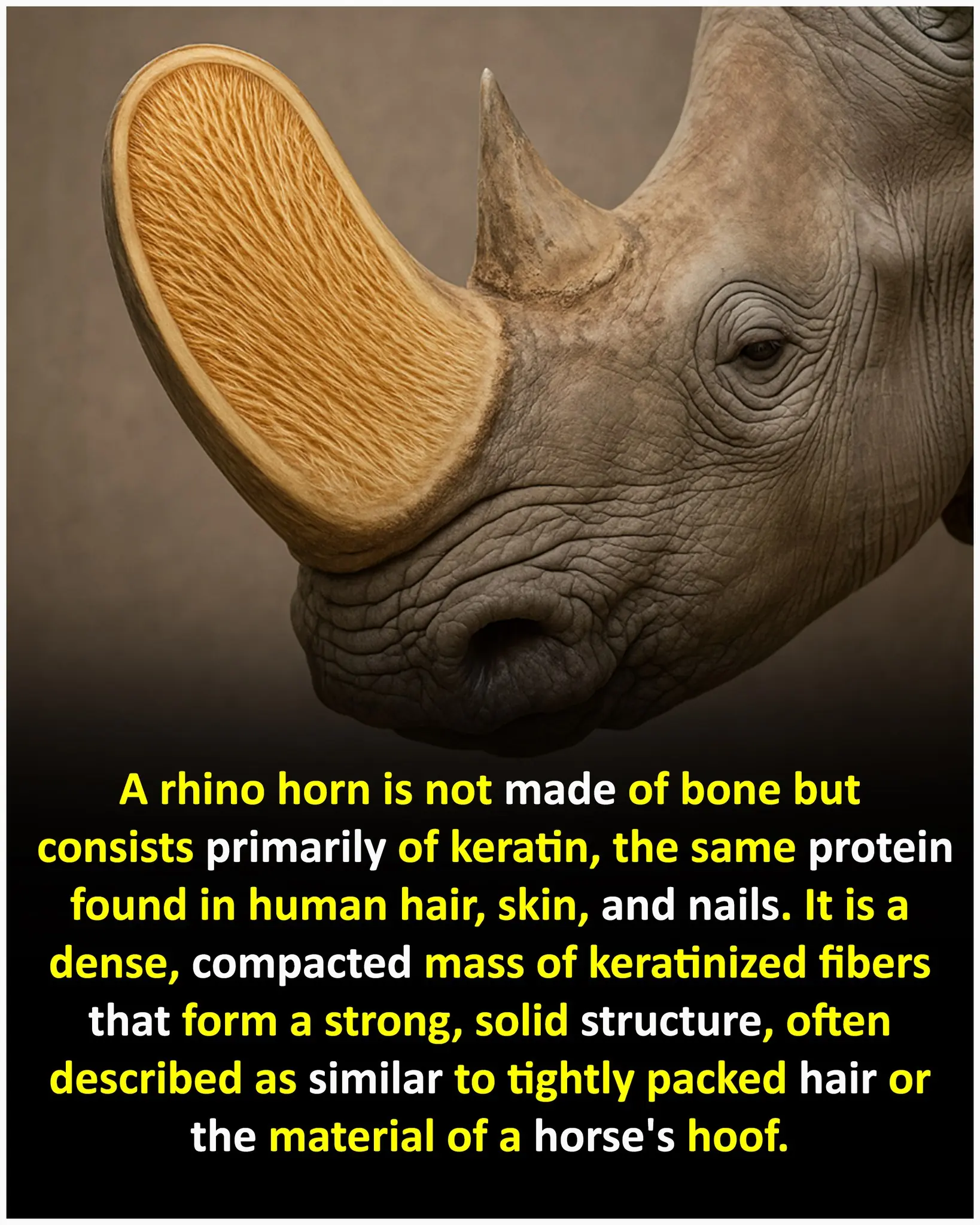
The Truth About Rhino Horns: What They're Really Made Of

Never keep these 4 relics after losing a loved one

A Touch of Viking Brilliance: Moss-Carpeted Homes in Norway
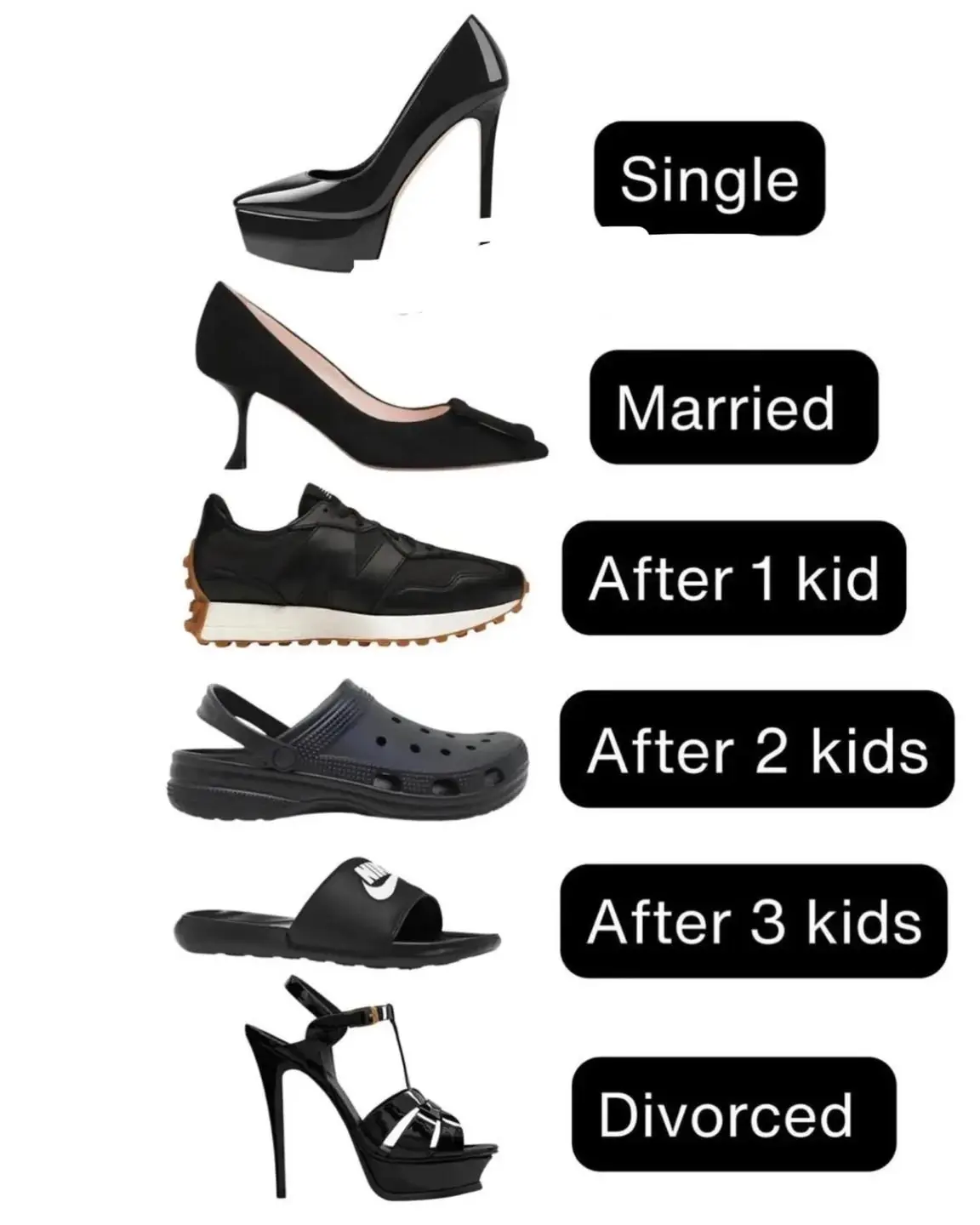
👠 From Stilettos to Slides: A Woman’s Life Journey Told Through Her Shoes

The ring you pick will reveal your truest trait

5 Reasons Why Some Men Prefer Slim Women

Why do women cross their legs when sitting?

16 Subtle Clues Your Partner May Not Be Loving You as You Deserve

The Fascinating History and Ingenious Design of Japan’s Nightingale Floors

What does it symbolize when a person who passed away appears in your dream
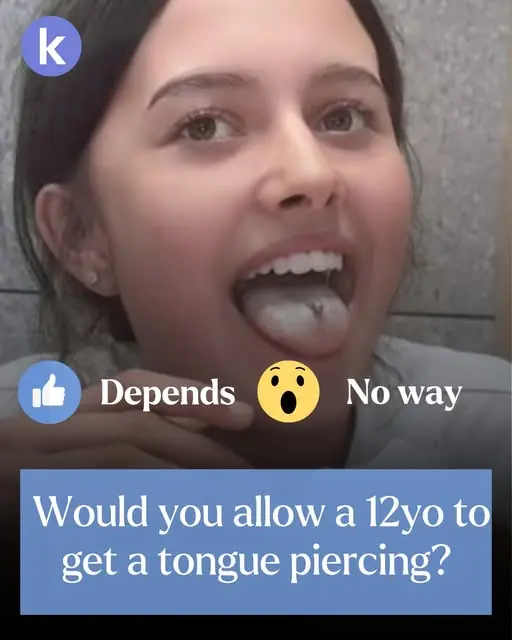
'Too sexual': I let my 12yo pierce this hidden body part

Photos From The Past That Changed The World”: Rare Historical Photos That Are Worth Seeing
The Fascinating History and Ingenious Design of Japan’s Nightingale Floors

JOKE OF THE DAY: A guy suspects his wife is cheating on him
Man in China Become Fluent in Japanese After Watching Over 4000 JAVS
News Post
9 ‘Classic’ Symptoms Signaling Early-Stage Cancer in Children: Parents Should Take Their Child to the Hospital Immediately

Heartfelt Advice: Throw Away These 4 Toxic Plastic Items Before Cancer Comes Knocking

NATURAL REMEDIES FOR INFLAMED GUT

😋💕 Mini Strawberry & Chocolate Party Cakes Recipe 💕😋

💕😋 Chocolate Cheesecake Cookies Recipe 😋💕

Extreme Freakshake S’mores Milkshakes: A Decadent Dessert Experience in a Glass 😋🔥🍫

Daily Baking Soda Intake May Help Tame Autoimmune Inflammation, Study Finds

Who Would You Give Your Seat to on the Bus? Your Answer Reveals Personality Insights

Enhance Your Bladder and Prostate Health with Pumpkin Seeds! 🌿🎃

Discover the Power of Black Cumin Seed: Your Ultimate Healing Remedy! 🖤🌿

Soothe Leg Pain and Rheumatism with This Natural Remedy

Discover the Health Benefits of Red Onion and Ginger Tonic
Brighten Your Smile with Carrot Tops: A Natural Home Remedy
Rejuvenate Your Intestines and Liver with the 3-Day Carrot Cleanse!

Revitalize Your Body with a Refreshing Parsley and Lemon Cleanse

Walnut Tea: Unlocking the Power of Nature for Your Health
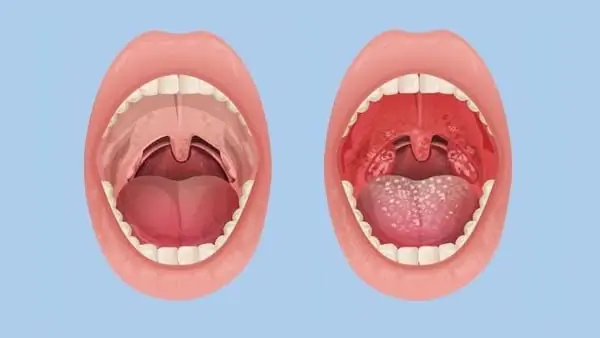
Many people mistake nasopharyngeal cancer for tonsillitis: Doctor explains how to tell them apart through associated symptoms

Ultimate Salted Caramel Cupcakes

Perfect Carrot Cake Cupcakes with Cream Cheese Frosting
