
Scientists Identify 380 Genetic Variants Responsible for Cancer

Researchers at Stanford University have identified 380 key genetic variants that play a significant role in cancer development.
Decoding Cancer's Genetic Risk
Thousands of small changes in the human genome’s DNA sequence have been linked to an increased risk of cancer. However, until now, it has been unclear which of these changes directly contribute to uncontrolled cell growth—the hallmark of cancer—and which are simply random coincidences or secondary factors.
Stanford researchers conducted the first large-scale analysis of these inherited genetic variations, known as single nucleotide variants (SNVs). Their study identified fewer than 400 variants that play a critical role in initiating and sustaining cancer development. These variants impact key biological mechanisms, including DNA repair regulation, energy production, and how cells interact with their microenvironment.
Common Biological Mechanisms in Cancer Growth
By pinpointing these common genetic factors, the findings could pave the way for new strategies to prevent or slow cancer development. Researchers also believe that this knowledge could enhance genetic screening, leading to better assessments of lifetime cancer risk.
The study, published on February 17, was led by doctoral researcher Laura Kellman and Paul Khavari, the Carl J. Herzog Professor at Stanford University School of Medicine and co-director of Stanford’s Epigenomics Program. Khavari, an elected member of the National Academy of Medicine, stated:
"We distilled an enormous dataset from millions of individuals diagnosed with any of the 13 most common cancers, which together account for over 90% of all human malignancies. This massive data funnel allowed us to identify 380 genetic variants that regulate the expression of one or more cancer-related genes. Some of these variants, if inherited from one’s parents, significantly increase the risk of multiple cancers."
Inherited Genetic Risks
The study focused on germline DNA sequences, which are inherited at conception, rather than mutations that accumulate throughout a person's life due to cell division and damage repair processes.
Well-known examples of inherited cancer-related mutations include BRCA1 and BRCA2, which greatly increase the risk of breast and ovarian cancer. However, only a few well-characterized mutations are currently used to predict cancer risk.
The variants identified by Kellman and Khavari are not found in protein-coding genes, which encode instructions for making the proteins that carry out most biological functions. Instead, they exist in regulatory regions, which control whether, when, and how much a gene is expressed. Typically, these regulatory regions influence nearby genes, though sometimes they can affect distant genes.
In 2020, Khavari launched a research initiative funded by the National Human Genome Research Institute to develop an Atlas of Regulatory Variants in Disease, aiming to identify variants linked to the risk of 42 complex diseases, including cancer. This project also seeks to personalize disease risk assessments to support screening, prevention, and new treatment strategies.
A New Approach to Understanding Cancer Risk
Previous studies, known as genome-wide association studies (GWAS), have identified genetic variants that appear more frequently in people with specific cancers than in those without cancer. However, these studies do not establish whether these variants alter gene activity in ways that increase or decrease cancer risk, nor do they identify which genes are affected.
Kellman, Khavari, and their colleagues took a different approach. They compiled over 4,000 suspected variants identified through GWAS data from 13 cancer types. By filtering through these thousands of potential variants, they narrowed it down to a few hundred functional regulatory regions.
Using available databases on DNA folding, tissue-specific gene expression, and other biological data, the researchers pinpointed approximately 1,100 target genes that likely play a role in cancer development. Some of these genes are specific to certain types of cancer, while others increase the risk of multiple cancers.
"Many of these genes align with our existing knowledge of cancer development," Khavari explained.
"Some genes regulate apoptosis (programmed cell death), while others influence how cells interact with their extracellular environment. One of the most striking examples involves mitochondrial function—tiny cellular powerhouses that support growth and division."
The Immune System’s Role in Cancer Development
However, the researchers also made unexpected discoveries.
"One particularly striking mechanism is the involvement of genes closely linked to inflammation," Khavari noted.
"While the connection between inflammation and cancer has been established, it remains unclear whether cancer cells or the immune system drive this process. Our findings suggest that interactions between cells and the immune system may contribute to chronic inflammation, increasing cancer risk."
Applying Gene Editing to Validate Findings
To further validate their discoveries, the researchers used gene-editing techniques on lab-grown cancer cells, demonstrating that nearly half of the identified variants were essential for continued cancer growth.
They hope their findings will serve as a foundation for researchers worldwide to better understand inherited cancer risk and develop new therapies.
"We now have the first-generation map of functional single nucleotide variants that define a person’s lifetime cancer risk," Khavari stated.
"We anticipate that this information will be integrated into increasingly sophisticated genetic screening tests over the next decade, helping identify individuals at risk for a wide range of complex genetic diseases, including cancer. This approach could enable personalized risk assessments and guide interventions, such as lifestyle modifications, pharmacological prevention, and targeted diagnostic screenings."
News in the same category


If Your Kidneys Are in Danger, Your Body Will Send You These 8 Signals — Don’t Ignore Them

The Surprising Effects of Avocado on Your Heart and Brain
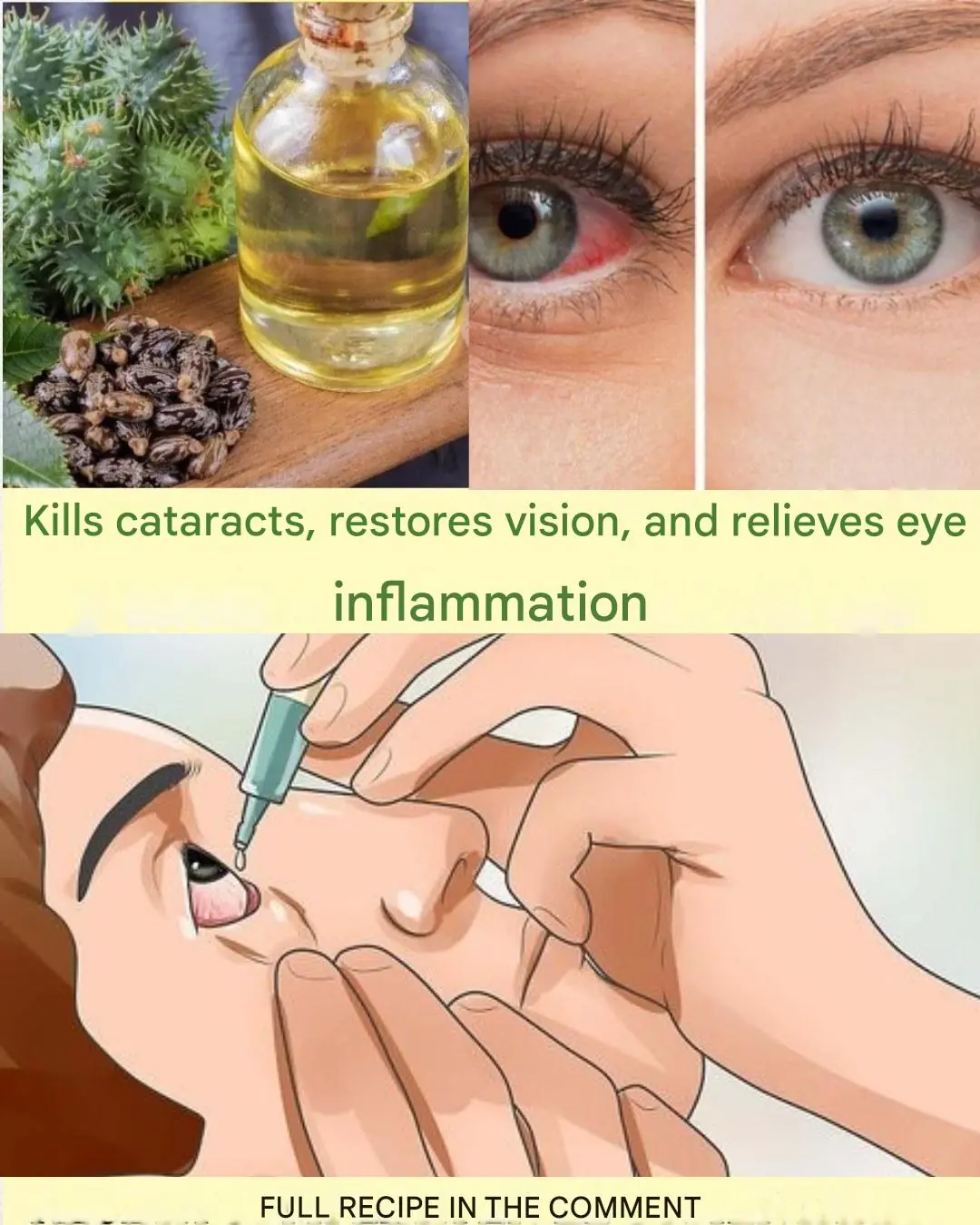
Natural Remedy for Cataracts and Eye Inflammation: Restore Your Vision Naturally

Unlock the Golden Magic of Corn Silk Tea
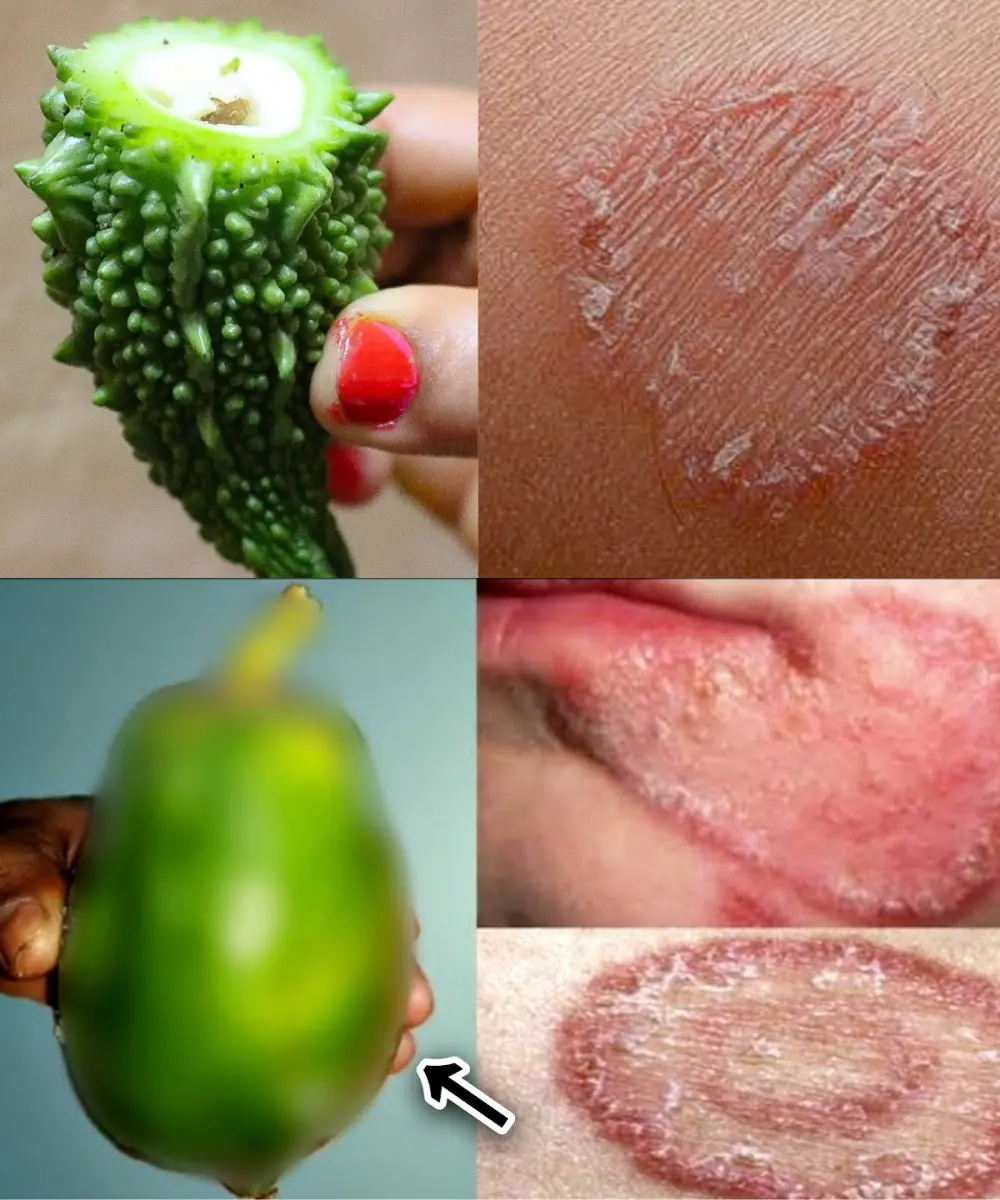
9 Powerful Home Remedies to Get Rid of Fungal Infection (Daad, Khaj, Khujli) Fast
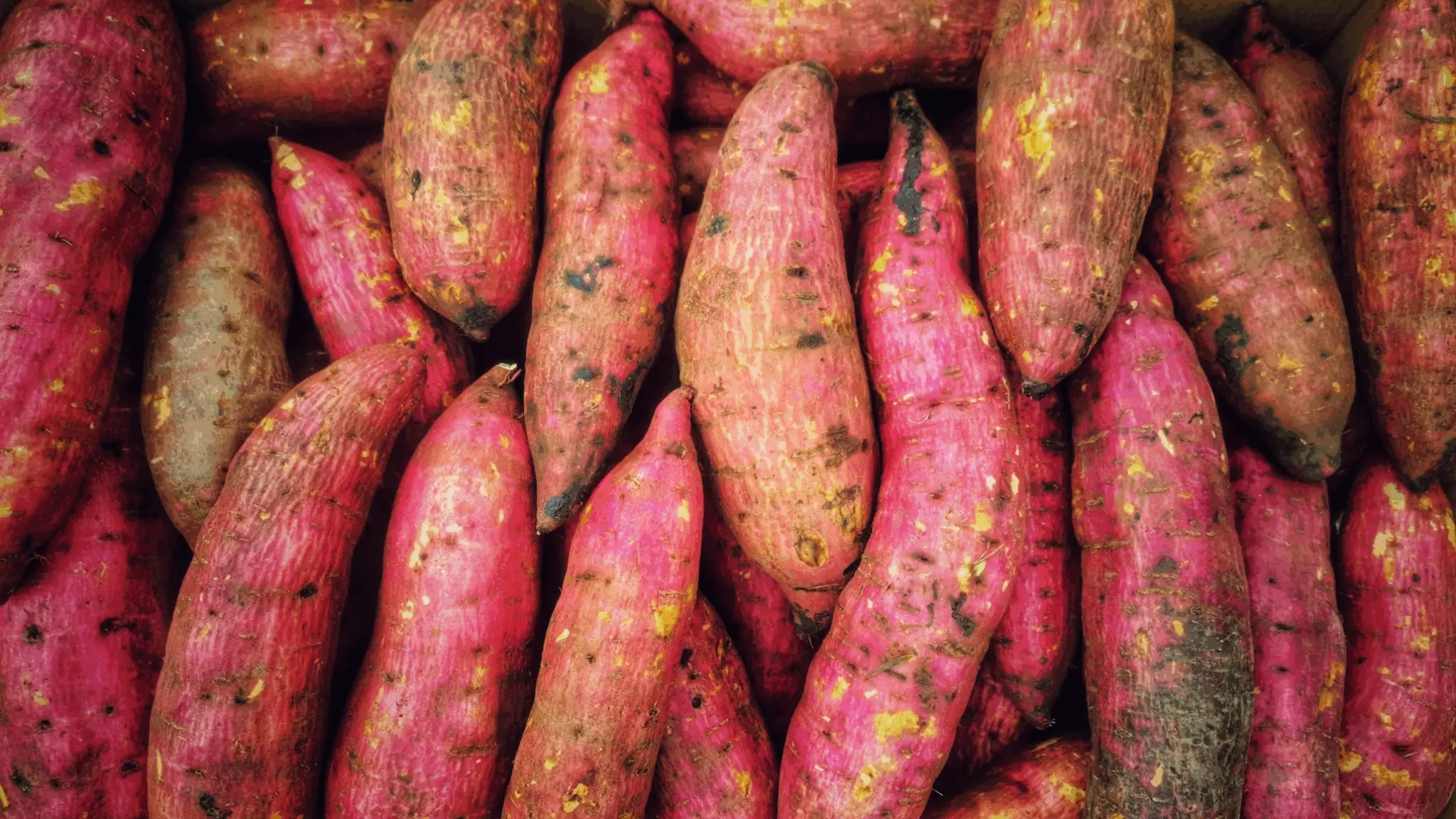
7 Shocking Health Benefits Of Eating Sweet Potatoes Every Day — According To Science

About 15 Minutes Before a Stroke, the Body Often Sends 4 Clear Warning Signs — Call Your Loved Ones Immediately
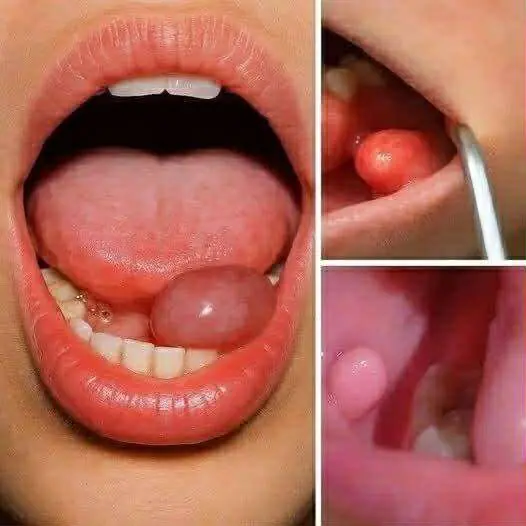
Hidden Dangers in Your Mouth: Early Signs of Oral Cancer

The Secret Power Of The Herb That Helps You Age Gracefully

The Unexpected Benefits of Eating Chicken Feet
If You See Someone with “Blue Veins,” Tell Them This — It Could Save Their Life
The Secret Power of Two Eggs a Day: Could This Simple Habit Transform Your Health? Buy vitamins and supplements

Man Passed Away After Eating Eggs — Stop Eating Eggs This Way Immediately
8 Foods That Fight Tumors — Eat Them Regularly

Does Eating Bananas Before Bed Have Any Benefits?

The Tongue as a Health Indicator: Meaning of a Whitish Color

Benefits of Boiled Eggs: Nutrition and Healthy Recipes
5 early warning signs of cervical cancer
7 Innocent Mistakes That Get Your Kidneys in Big Trouble
News Post
WHAT HAPPENS WHEN WE TONGUE KISS…See more

Nature’s Secret: 4 Healing Leaves That Support Metabolism, Immunity & Circulation Naturally

Don’t Drink Coconut Water Before You Know These 11 Secrets!

Pumpkin Seed Milk — The Natural Parasite Cleanser
Fast Rice Water Trick for a Brighter Smile
Morning Drink to Revive Your Kidneys Fast
The Onion Recipe That Could Transform Your Blood Sugar, Support Cleaner Arteries, and Protect Your Heart!

Top 4 Fruits That Help Your Kidneys Flush Out Toxins While You Sleep
Ginger, Clove, and Honey: The Natural Trio Your Body Will Thank You For

Heal 15 Years of Joint Pain Naturally with Turmeric and Honey Tea

This Juice Revived My Grandma’s Energy — Say Goodbye to Fatigue and Body Pain with This Natural Recipe

The Benefits of Eating 2 Boiled Eggs Every Morning: Transform Your Health!

If Your Kidneys Are in Danger, Your Body Will Send You These 8 Signals — Don’t Ignore Them

The Surprising Effects of Avocado on Your Heart and Brain

Ways to Get Over a Man Who Didn’t Value You

I’m 66 but Look 36 — My Secret? Aloe Vera & Ginger for Firm, Smooth Skin

How to Make Okra Water to Treat 17 Health Problems Naturally

Banana and Egg Mask to Look Younger Even in Your 80s

Scent Leaf Secrets Unveiled: 10 Surprising Health Benefits of This Miracle Herb
